About us
Beijing JWGB Instrument Co., Ltd.
sales@jwgb.net
Copyright 2004–2024 JWGB.NET
 TEL: +86 010 63326036
TEL: +86 010 63326036
 ENAIL:sales@jwgb.net
ENAIL:sales@jwgb.net
Chemisorption, the chemical bonding between gas-phase molecules andsurface atoms, is the first and most important step in a catalytic reaction On supported metal catalysts, chemisorption takes place on small metal crystallites, which are typically anchored to a high surface area oxide material. These chemisorbed molecules then react with other adsorbed species or with gas-phase molecules to produce reaction products. Why is chemi-sorption so important? The rate of the catalytic reaction as well as its selectivity to desired products is directly related to the chemisorption properties of the supported metal catalyst.
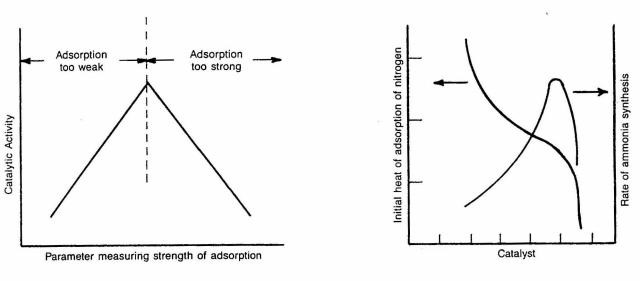
Strength of chemisorption is only one of the parameters affecting catalytic activity. The number of chemisorbed molecules is also important. It follows that more chemisorbed species means more reaction product molecules. The number of molecules forming chemisorption bonds with the catalyst surface is related to the number of surface atoms available for bonding. Maximizing the number of these surface "sites" for chemisorption is a top priority in good supported metal catalyst design.
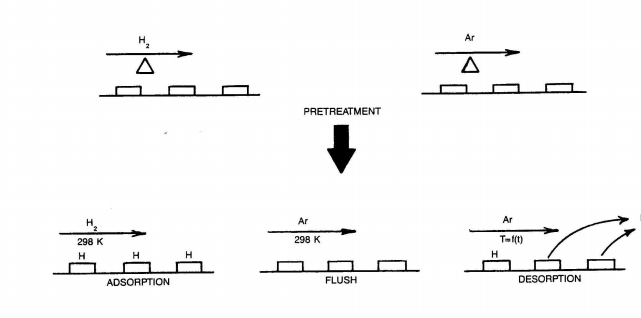
How can we measure these important parameters? We need a technique which can count the number of chemisorption sites and tell us something about how tightly these sites hold onto chemisorbing molecules. A chemisorbing molecule which binds with a known stoichiometry to a surface site can act as our probe of the surface. For supported metal catalysts, this "selective chemisorption" of molecules on metal surface sites can be monitored by:
-measuring the equilibrium uptake of gas phase molecules by the surface in a closed system termed "static" or "volumetric" chemisorption
-detecting how many calibrated pulses of chemisorbing molecules are taken up by the surface (pulse chemisorption)
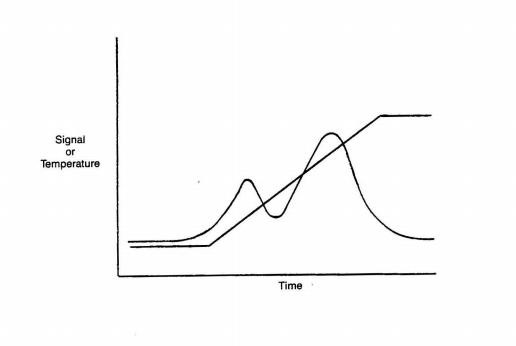
The first two techniques are performed under isothermal (most often ambient temperature) conditions and hence can offer little information about adsorption strength. The third technique, one in a family of temperature- programmed techniques for catalyst characterization, can provide information about both of the parameters of importance: strength and number of chemi- sorbing sites.